Commonly Tested Lewis Structures
A step-by-step explanation of how to draw the AlH3 Lewis Structure. For the AlH3 Lewis structure use the periodic table to find the total number of valenc. Drawing Lewis Structures.
- Read my article in Science Education based on my dissertation.
We draw Lewis Structures to predict:
-the shape of a molecule.
-the reactivity of a molecule and how it might interact with other molecules.
-the physical properties of a molecule such as boiling point, surface tension, etc.
Lewis Dot Structure Calculator
Video: Drawing the Lewis Structure for ICl4-
For the ICl4- Lewis structure the total number of valence electrons (found on the periodic table) for the ICl4- molecule. Once we know how many valence electrons there are in ICl4- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
In the Lewis structure of ICl4- there are total of 36 valence electrons.
Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons.
Lewis Dot Structure Calculator Math
Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
It is helpful if you:
- Try to draw the ICl4- Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to ICl4- for more practice.
List of Lewis Structures
| Acetone | BF3 | BH4- | BrF5 | BrO3- | C2H2 | C2H4 | ClO- |
| CH4 | ClO2- | ClO2 | ClO4- | CO | CS2 | H2O | H3O+ |
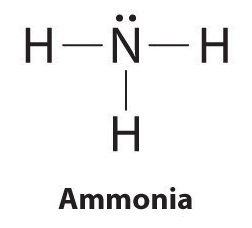
| HCl | HNO3 | I3- | ICl4- | IF5 | N2 | N3- | NH2OH |
| NH3 | NO2- | NO2 | NO3- | O2 | OF2 | PCl5 | PH3 |
| PO33- | PO43- | SCl2 | SF4 | SF6 | SO3 | SO42- | XeF2 |
| XeF4 |
/lewis-fc84e3f1452e4aacb2fe023cfff2fa08.jpg) Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice.
Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict:
Lewis Dot Structure Generator
- the shape of a molecule.
- how the molecule might react with other molecules.
- the physical properties of the molecule (like boiling point, surface tension, etc.).
That helps us understand and predict interactions with things like medicine and our body, materials used to make buildings and airplanes, and all sorts of other substances. Lewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative.
Search 100+ Lewis Structures on our site.
(Opens new window.) |

Click the Chemical Formula to see the Lewis Structure | Acetone | (C3H6O) | | AsCl3 | (Arsenic Trichloride) | | AsF3 | (Arsenic Trifluoride) | | AsF5 | (Arsenic Pentafluoride) | | AsF6- | (AsF6-) | | AsH3 | (Arsenic Trihydride) | | AsO33- | (Arsenite Ion) | | BBr3 | (Boron Tribromide) | | BCl3 | (Boron Trichloride) | | BF3 | (Boron Trichloride) | | BF4- | (Tetrafluoroborate Ion) | | BH3 | (Boron Hydride) | | BH4- | (BH4-) | | B(OH)3 | (B(OH)3) | | BeCl2 | (Beryllium Chloride) | | BeF2 | (Beryllium Fluoride) | | BeH2 | (Beryllium Hydride) | | Br2 | (Bromine Gas or Elemental Bromine) | | Br3- | (Tribromide Ion) | | BrF | (Bromine Monofluoride) | | BrF2 | (Bromine Difluoride) | | BrCl3 | (Bromine Trichloride) | | BrF3 | (Bromine Trifluoride) | | BrF5 | (Bromine Pentafluoride) | | BrO- | (Hypobromite Ion) | | BrO2- | (Bromite Ion) | | BrO3- | (Bromate Ion) | | C22- | (Dicarbide Ion) | | CBr4 | (Carbon Tetabromide) | | CCl4 | (Carbon Tetachloride) | | ClF | (Chlorine Monofluoride) | | CF2Cl2 | (Dichlorodifluoromethane) | | CH2Cl2 | (CH2Cl2) | | CH3- | (CH3-) | | CH3Br | (CH3Br) | | CH3Cl | (Chloromethane or Methyl Chloride) | | CH3CN | (Acetonitril or Methyl Cyanide) | | CH3COO- | CH3COO- | | CH3COOH | (Acetic Acid) | | CH3F | (CH3F) | | CH3NH2 | (Methylamine) | | CH3NO2 | (CH3NO2) | | CH3OCH3 | (Dimethyl Ether or Methoxymethane) | | CH3OH | (Methanol or Methyl Alcohol) | | CH4 | (Methane) | | C2F4 | (C2F4) | | C2H2 | (Ethyne or Acetylene) | | C2H2Br2 | (C2H2Br2) | | C2H2Cl2 | (C2H2Cl2) | | C2H4 | (Ethene) | | C2H6 | (Ethane) | | C2H6O | C2H6O | | C3H6 | (C3H6) | | C3H8 | (Propane) | | C4H10 | (Butane) | | C6H6 | (Isomers - including Benzene) | | C6H12 | (C6H12) | | CHCl3 | (Chloromethane) | | CH2F2 | (Difluoromethane) | | CH2O | (Methanal or Formaldehyde) | | CH4O | (CH4O) | | Cl2 | (Chlorine Gas or Elemental Chlorine) | | Cl2CO | (Cl2CO) | | Cl2O | (Dichlorine Monoxide) | | Cl3PO | (Phosphoryl Trichloride) | | ClF3 | (Chlorine Trifluoride) | | ClF5 | (Chlorine Tetrafluoride) | | ClO- | (Hypochlorite Ion) | | ClO2 | (Chlorine Dioxide) | | ClO2- | (Chlorite Ion) | | ClO3- | (Chlorate Ion) | | ClO4- | (Perchlorate Ion) | | CO | (Carbon monoxide) | | CO2 | (Carbon Dioxide) | | CO32- | (Carbonate Ion) | | COCl2 | (COCl2) | | COF2 | (COF2) | | COH2 | (COH2) | | CN- | (Cyanide Anion) | | CS2 | (Carbon Disulfide) | | F2 | (Fluorine Gas, Difluorine) | | H2 | (Hydrogen Gas or Elemental Hydrogen) | | H2CO | (Formaldehyde or Methanal) | | H2CO3 | (Carbonic Acid) | | H2O | (Water or Dihydrogen monoxide) | | H3O+ | (Hydronium Ion) | | H2O2 | (Hydrogen Peroxide or Dihydrogen Dioxide) | | HBr | (Hydrogen Bromide or Hydrobromic Acid) | | HF | (Hydrogen Fluoride or Hydrofluoric Acid) | | HCCH | (Ethyne) | | HCl | (Hydrogen Chloride or Hydrochloric Acid) | | HCO2- | (Formate Ion) | | HCO3- | (Hydrogen Carbonate Ion or Bicarbonate Ion) | | HCOOH | (Methanoic Acid or Formic Acid) | | HI | (Hydrogen Iodide or Hydroiodic Acid) | | HClO3 | (Chloric Acid) | | HCN | (Hydrogen Cyanide) | | HNO2 | (Nitrous Acid) | | HNO3 | (Nitric Acid) | | H2S | (Dihydrogen Sulfide) | | HOCl | (Hypochlorous Acid) | | H2Se | (Dihydrogen Selenide) | | HSO3- | (Bisulfite Ion) | | HSO4- | (Bisulfate Ion) | | H2SO3 | (Sulfurous Acid) | | H2SO4 | (Sulfuric Acid) | | H3PO4 | (Phosphoric Acid) | | I2 | (Iodine Gas or Elemental Iodine) | | I3- | (I3-) | | IBr2- | (IBr2-) | | ICl | (Iodine Chloride) | | ICl2- | (ICl2-) | | ICl3 | (ICl3) | | ICl4- | (ICl4-) | | ICl5 | (Iodine Pentachloride) | | IF2- | (IF2-) | | IF3 | (Iodine Trifluoride) | | IF4- | (IF4-) | | IF5 | (Iodine Pentafluoride) | | IO3- | (Iodate Ion) | | IO4- | (Perioiodate Ion) | | N2 | (Nitrogen Gas, also called Elemental Nitrogen) | | N3- | (Azide Ion) | | N2F2 | (Dinitrogen Difluoride) | | N2H2 | (Dinitrogen Dihydride) | | N2H4 | (Dinitrogen Tetrahydride or Hydrazine or Diamine) | | N2O3 | (Dinitrogen Trioxide) | | N2O4 | (Dinitrogen Tetroxide) | | N2O5 | (Dinitrogen Pentoxide) | | NCl3 | (Nitrogen Trichloride) | | NF3 | (Nitrogen Trifluoride) | | NH2- | (NH2-) | | NH2Cl | (Chloroamine) | | NH2OH | (Hydroxylamine) | | NH3 | (Ammonium or Nitrogen Trihydride) | | NH4+ | (Ammonium Ion) | | NI3 | (Nitrogen Triiodide) | | NO+ | (Nitrosonium Ion) | | NO | (Nitric Oxide or Nitrogen Monoxide) | | N2O | (Nitrous Oxide or Dinitrogen Monoxide) | | NO2 | (Nitrogen Dioxide) | | NO2- | (Nitrite Ion) | | NO2Cl | (NO2Cl) | | NO2F | (NO2F) | | NO3- | (Nitrate Ion) | | NOBr | (Nitrosyl Bromide) | | NOCl | (Nitrosyl Chloride) | | NOF | (Nitrosyl Fluoride) | | O2 | (Oxygen Gas, also called Elemental Oxygen) | | O22- | (Perioxide Ion) | | O3 | (Ozone) | | O3 | O3 Resonance Structures | | OCl2 | (OCl2) | | OCN- | (Cyanate Ion) | | OCS | (OCS) | | OF2 | (Oxygen Difluoride) | | OH- | (Hydroxide Ion) | | PBr3 | Phosphorus Tribromide | | PBr5 | Phosphorus Pentabromide | | PCl3 | Phosphorus Trichloride | | PCl4- | PCl4- | | PCl5 | Phosphorus Pentachloride | | PF3 | Phosphorus Trifluoride | | PF5 | Phosphorus Pentafluoride | | PF6- | Hexafluorophosphate Ion | | PH3 | Phosphorus Trihydride | | POCl3 | Phosphoryl Chloride or Phosphorus Oxychloride | | PO33- | (Phosphite Ion) | | PO43- | (Phosphate Ion) | | SBr2 | (Sulfur Dibromide) | | SCl2 | (Sulfur Dichloride) | | SCl4 | (Sulfur Tetrachloride) | | SCN- | (Thiocyanate) | | SeF4 | (Selenium Tetrafluoride) | | SeF6 | (Selenium Hexafluoride) | | SeO2 | (Selenium Dioxide) | | SF2 | (Sulfur Difluoride) | | SF4 | (Sulfur Tetrafluoride) | | SF6 | (Sulfur Hexafluoride) | | S2Cl2 | (Diulfur Dichloride) | | SiCl4 | (Silicon Tetrachloride) | | SiF4 | (Silicon Tetrafluoride) | | SiF62- | (Silicon Hexafluoride Ion) | | SiH4 | (Silicon Tetrahydride) | | SiO2 | (Silicon Dioxide) | | SnCl2 | (Tin (II) Chloride) | | SOCl2 | (SOCl2) | | SO2 | (Sulfur Dioxide) | | SO3 | (Sulfur Dioxide) | | SO32- | (Sulfite Ion) | | SO42- | (Sulfate Ion) | | Water | (H2O) | | XeCl4 | Xenon Tetrachloride | | XeF2 | XeF2 | | XeF4 | Xenon Tetrafluoride | | XeF6 | Xenon Hexafluoride | | XeH4 | XeO4 | | XeO3 | XeO3 | | XeO2F2 | XeO2F2 |
| Steps for Writing Lewis Structures - Find the total valence electrons for the molecule. Explain How Examples: H2S, NCl3, OH-
- Put the least electronegative atom in the center.
Note: H always goes outside.
Examples: NOCl, CF2Cl2, HCN
- Put two electrons between atoms to form a chemical bond. Examples: CH4, NH3, I2
- Complete octets on outside atoms.
Note: H only needs two valence electrons.
- If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
Examples: O2, N2, C2H4
Advanced Steps - If you have extra electrons after the above steps add them to the central atom. Note: elements in the Period Three (usually S, P, or Xe) can have more than eight valence electrons.
Examples: ClF3, SF4,XeH4
- Check the Formal Charges to make sure you have the best Lewis Structure. Explain How
Examples: SO42-, N2O, XeO3
Notable Exceptions to the Octet Rule - H only needs 2 valence electrons.
- Be and B don’t need 8 valence electrons.
- S and P sometimes have more than 8 val. Electrons.
- Elements in Period Three, Four, etc (on the periodic table) can hold more than 8 valence electrons.
|


/lewis-fc84e3f1452e4aacb2fe023cfff2fa08.jpg)

Comments are closed.